Technology
Nanoparticle Drug Delivery
With the ever-increasing importance and prevalence of nanoparticle-based treatments like mRNA vaccines, the relevance of nanomedicines is only accelerating.
In order to produce these new medical treatments in an efficient manner, the pharmaceutical industry has had to re-evaluate outdated existing manufacturing processes in favor of state-of-the-art technologies like those innovated by DIANT® Pharma.
mRNA-LNP Technology

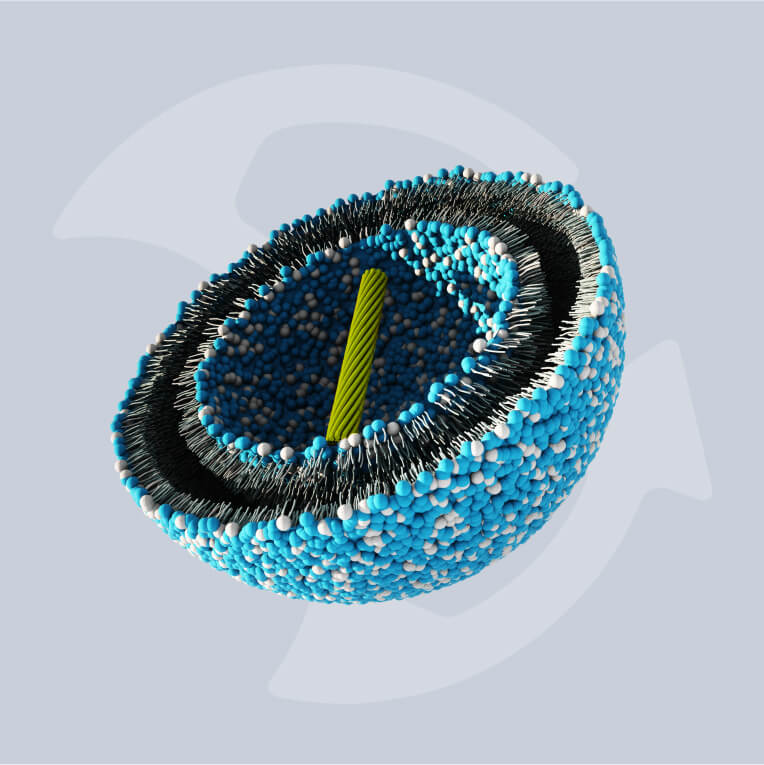
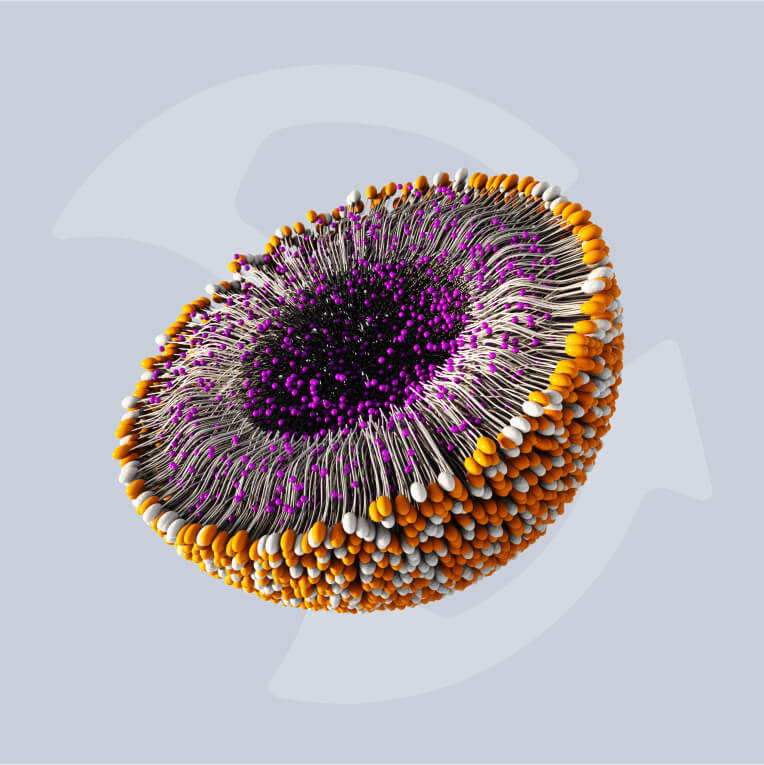
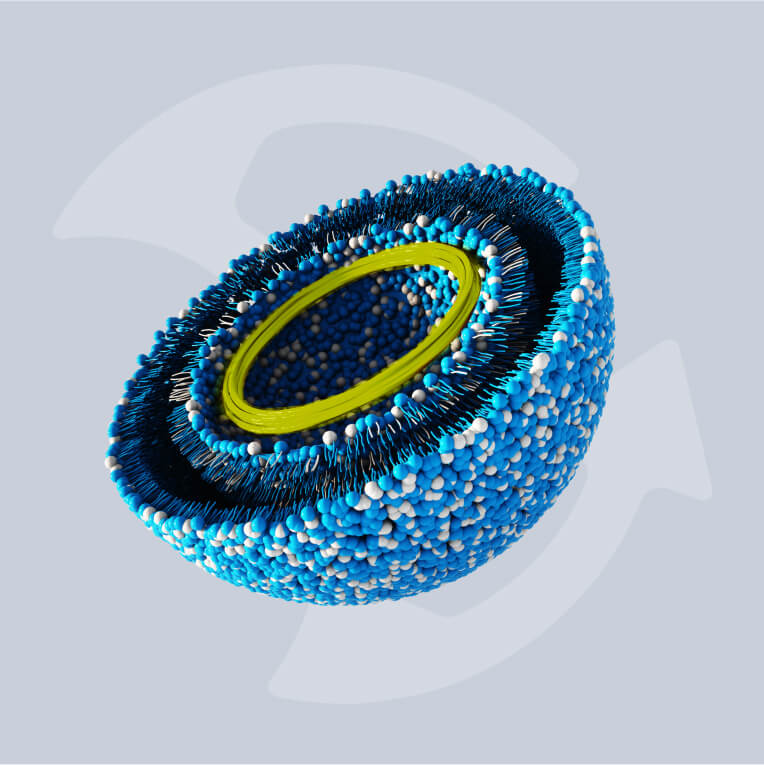
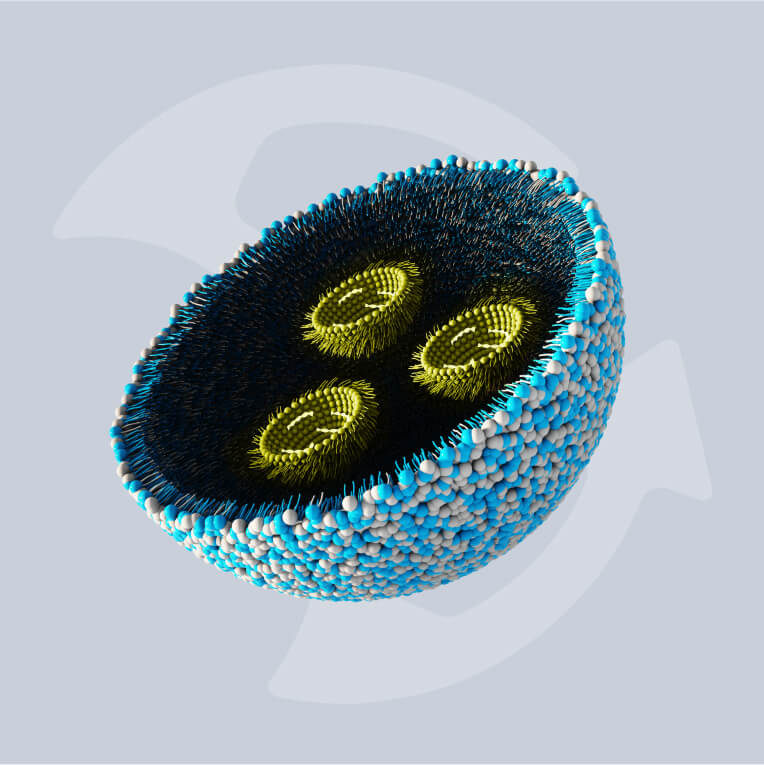
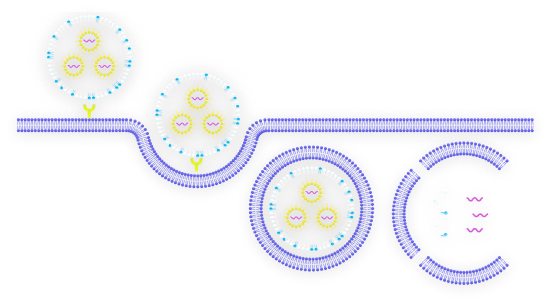
Lipid nanoparticles (LNPs) have become the delivery technology of choice for nanomedicines. They offer the necessary efficacy and production efficiency for a new generation of increasingly complex therapeutics, vaccines, and oncology treatments.
The advancement of LNP-based messenger RNA (mRNA) vaccines in the last decade has changed the face of immunology and heralded the dawn of a new age in biomedical manufacturing. LNP therapeutics like mRNA vaccines demonstrate a high efficacy compared with traditional medicines but require modernized methods of manufacture in order to be produced cost-effectively at scale.
With numbers of these mRNA treatments, including therapeutics based in gene editing, growing in both clinical trials and on the market, updating these modes of production has become more pressing than ever. Increasing drug complexity necessitates new technologies that can successfully encapsulate active pharmaceutical ingredients (APIs) to support efficacy.
When combined with process analytical technology (PAT) to control for particle size and other analytical parameters during manufacture, technology like the DIANT® Jet can hasten nanomedicine production while also minimizing the risk inherent in older batch processing biomanufacture.
Biotargeting
As medicine is becoming increasingly specialized and demand for treatments targeted to individual patients and rare diseases emerge, the mechanisms by which these treatments work have naturally had to change. This has led to the development of various biotargeted nanoparticles to diagnose or treat both common and complex conditions.
Biotargeted nanomedicines are intravenously injected nanoparticles that distribute a drug or imaging agent into the body in a targeted manner. They can target specific organs and tissues all the way down to cells and sub-cellular compartments.
These medicines have emerged as promising diagnostics and treatments for certain cancers and even brain injuries. It’s clear that the future of medicines based on the targeted deployment of liquid drugs is promising, with long-acting targeted injectables also on the horizon.
Particle Engineering
Changes in cGMP biopharmaceutical manufacturing have accompanied the emergence of biotargeted nanomedicines, including developments in particle engineering.
Multiple properties of nanoparticles need to be controlled in order to develop effective, non-invasive treatments and diagnostics. The size, shape, chemistry, surface properties, and structure of nanoparticles are just a few properties that determine their bioavailability, therapeutic potential, and ultimately their performance in medicines and other applications.
Naturally, controlling these properties in engineered nanoparticles (ENPs) is becoming a major priority in pharmaceutical manufacturing in order to ensure the quality and efficacy of these new nanomedicines and other nanoparticle products.
The use of closed system nanoparticle manufacturing like DIANT’s proprietary process has been shown to greatly help manage these qualities by automatically controlling particle size and limiting the necessity for human involvement during the manufacturing process.